
What You Missed
As Lecanemab, Tofersen, and Donanemab brought us into a new era of neuroscience breakthroughs in 2024, and SV95C is heralded as the first-ever regulatory-qualified digital biomarker, now is the time to capitalize on objective, more quantitative, and less invasive digital measures to reduce costs and improve patient experience.
With innovations in speech, cognition, sleep, motor, facial, and eye tracking identified as clinically relevant measures across a breadth of CNS indications from neurodegenerative, neuromuscular, psychiatric, and neurodevelopmental indications and epilepsies, we’re just scratching the surface of the expansive potential of digital measures in neurology.
The 3rd Digital Biomarkers & Clinical Measures for Neurology Summit welcomed 25+ key opinion leaders to the podium in August, discussing the implementation of these measures into their clinical trials, clinical development, regulatory approach, commercialization, and reimbursement strategy.
Digital Tools to Accelerate the Pipeline to Effective & Safe CNS Drugs

Navigating global regulatory pathways for digital biomarker qualification, precompetitive collaboration and standardization with Novartis, Merck, DEEP Measures, and Roche
Harness wearable devices for longitudinal monitoring of patient disease burden and improvement across epilepsies, neurodevelopmental and neuromuscular disorders with Takeda, Alexion Pharmaceuticals, Coalition of Advancing Science, and Novartis
Evaluating multifactorial measures of speech across ALS, Psychiatry and Parkinson’s Disease for longitudinal monitoring of disease with Boehringer, and Mitsubishi Tanabe Pharma

Explore real-world approaches to digital therapeutics for mental health with BMS, Boehringer Ingelheim, and Cerevel Therapeutics and cognitive impairment for earlier diagnosis and more accurate quantification of disease burden with Janssen, Regeneron, and South Shore Hospital

Delve into the commercialization of digital biomarkers in a real-world payer and reimbursement landscape with Pfizer, Alexion, and Harvard Medical School while prioritizing clinical relevance, usability, and patient perspectives with Biogen and Eisai
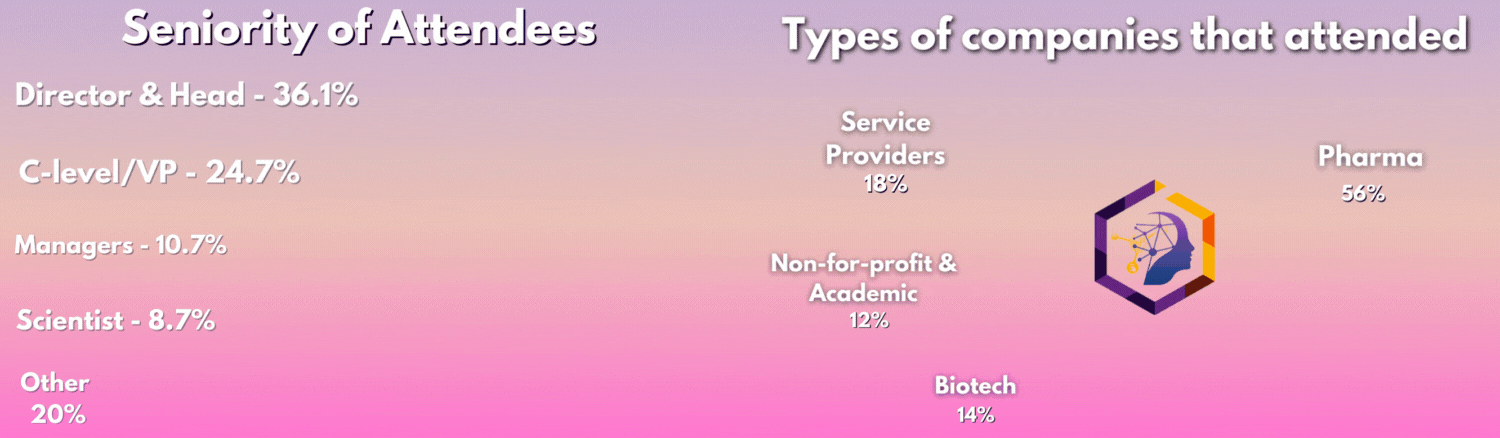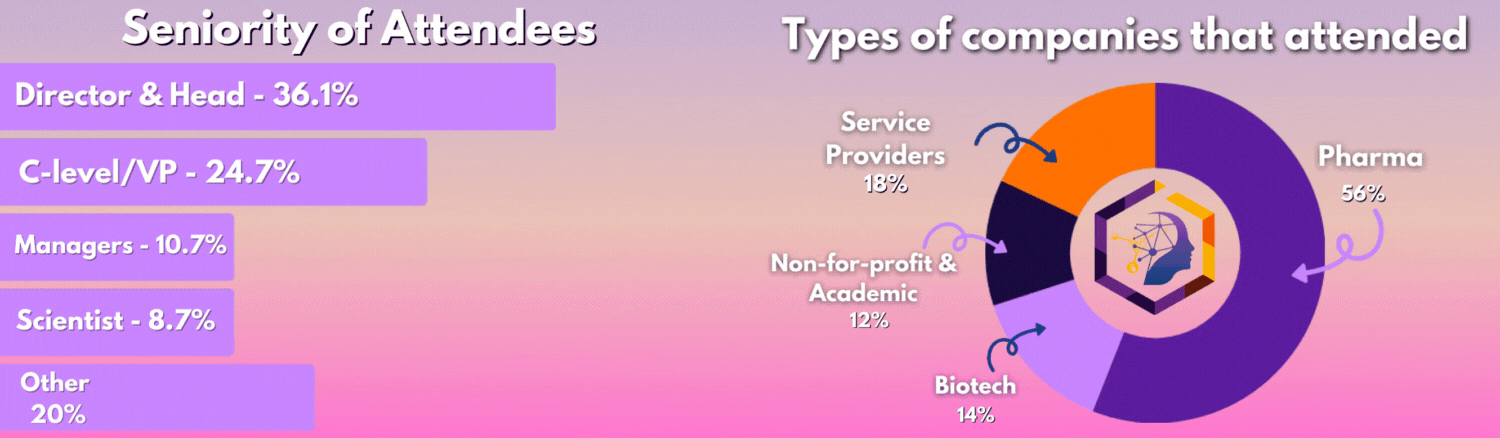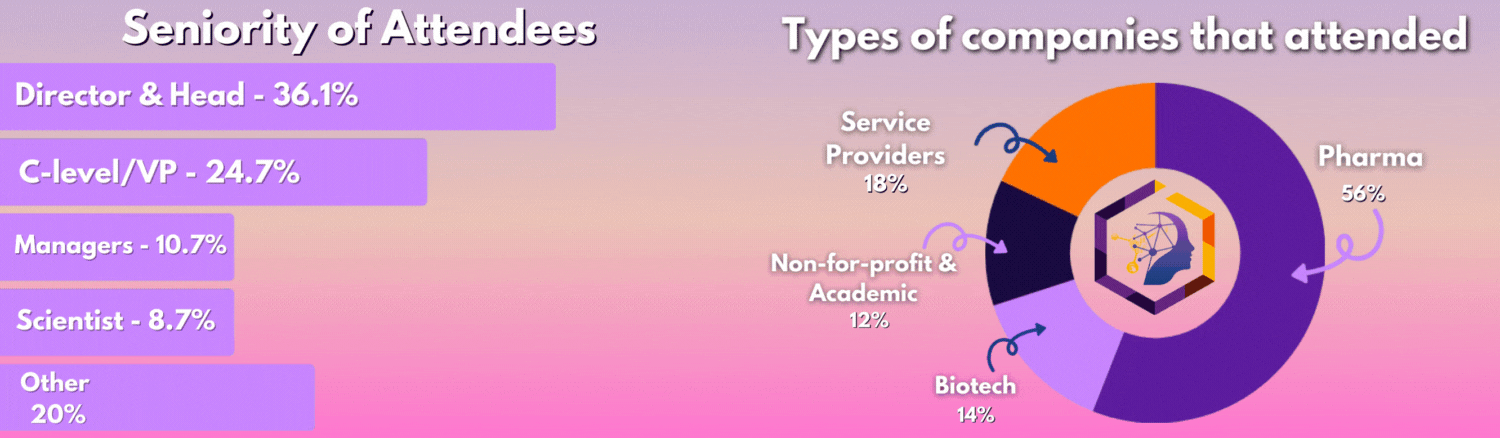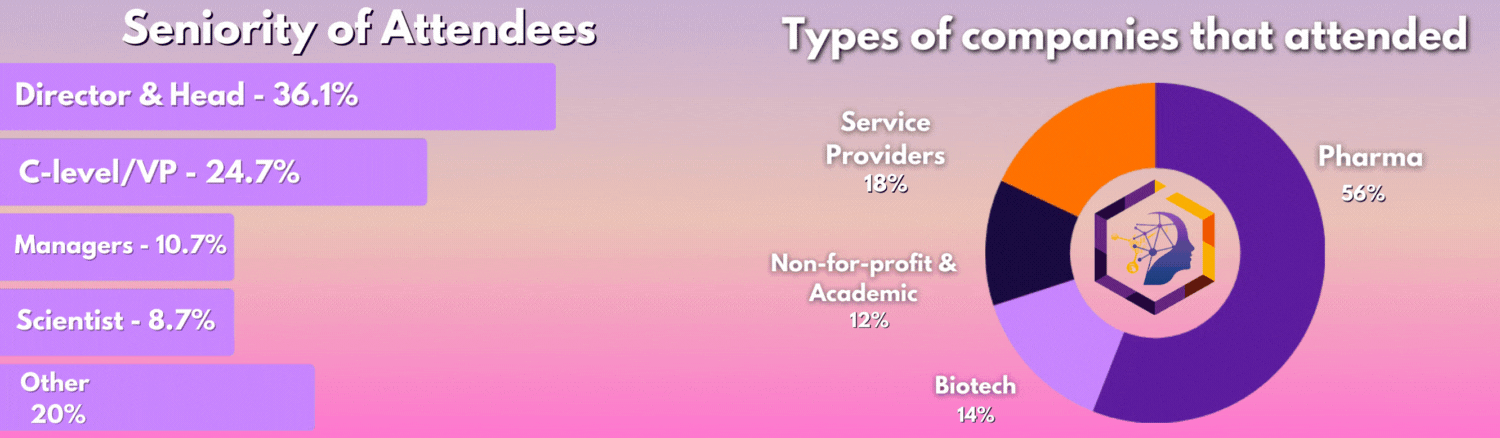
Who Was In The Room?
The 3rd Digital Biomarkers & Clinical Measures for Neurology meeting united 50+ key pharma and biotech leaders across translational medicine, clinical development, digital health, medical affairs, and regulatory affairs.
From C-Level executives to clinical research scientists, the leading industry figures united this August to discover the latest unpublished data, hear heated panel discussions, and join deep-dive workshops you can't find anywhere else.
What Your CNS Digital Health Peers Had to Say:






